Liquid and Gas Separations
An emerging area of research is on material discovery with prime focus to rational design of membranes for gas and liquid separation applications. Computer simulations has the great potential to help experiments in that regard. The reason is in-silico predictions of gas and liquid adsorption has been proven to be quite reliable. Reliability of course is measured how close the computer-based value of the observable is compared against experimental one. Another advantage is that due to the advances in the software and hardware technologies high throughput data generation is seamlessly easy in contrast to experiments. Making computational studies an important component of modern material science.
Atomically detailed simulations can provide features with little effort, making the computer simulations as ideal source for computer-based learning for material discovery. This opens doors for artificial intelligence and machine learning applications that we utilize in our lab.
Gas separations
In addition, molecular simulations help to uncover the fundamental principles of fluid transport through membranes and develop novel computational and physical insights that will facilitate the design of potent membranes for oil and gas industry.
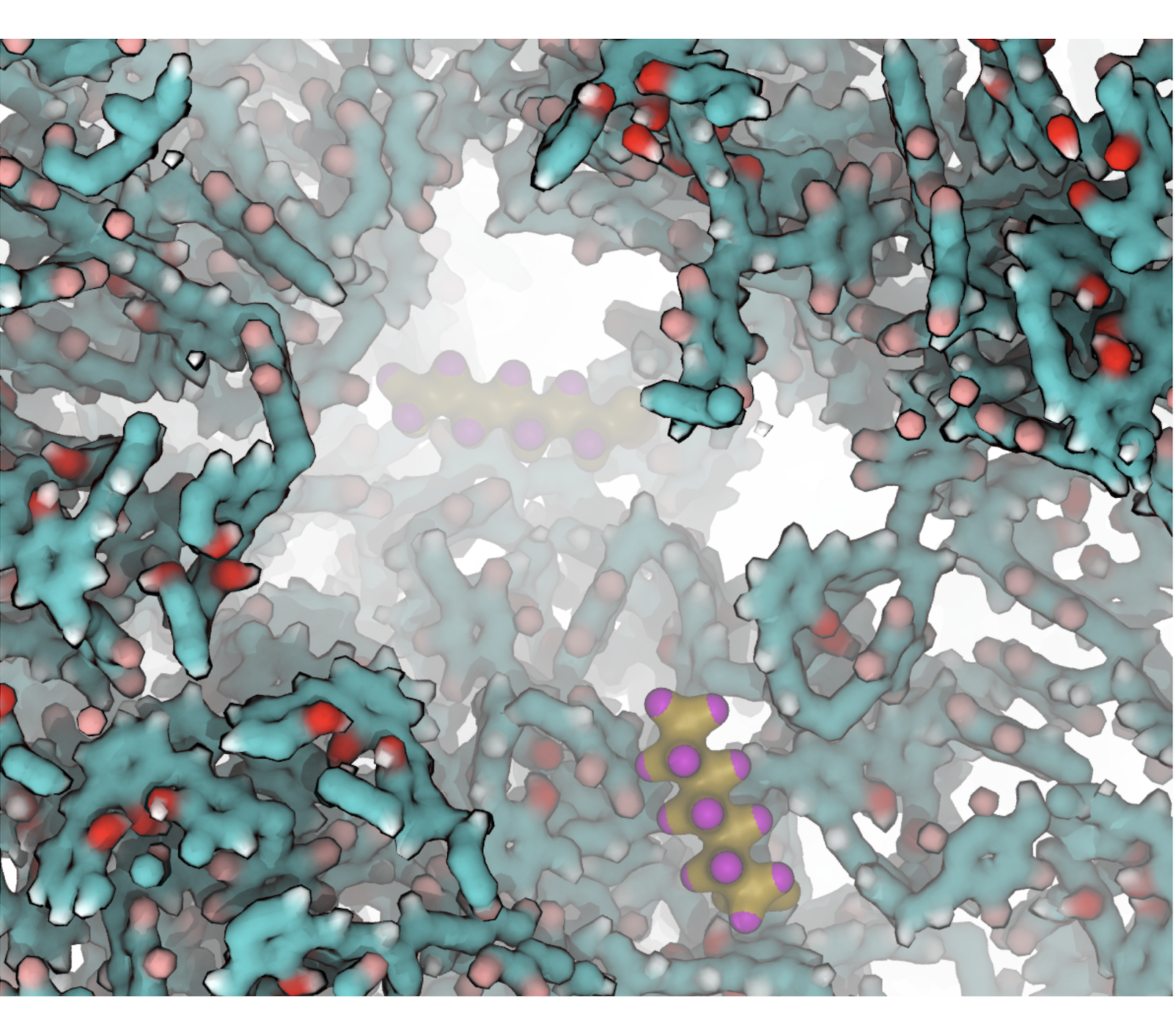
To achieve our goal, we use quantum mechanics to determine the structure and interactions. Molecular dynamics simulations assess local fluctuations of the materials in thermal energy. Monte Carlo simulations allow computing the observables such as uptake ratios, selectivity and permeability. Fig 1 shows I 2 binding isotherms obtained from Grand Canonical Monte Carlo simulations (1-2).
Of course, computational predictions always need to be benchmarked. For that purpose, we collaborate with experimental groups. Most notable of them are Prof. Ali Trabolsi Lab. and Prof. Jagannathan Lab. Our focus is on Calixerene based polymers forming 2 and 3dimensional networks (1-2).
Water treatment
Another direction is aimed to develop further insights and technologies for water treatment. Using computer simulations, we study how toxic materials can be removed from water. Fig 2 is a snapshot from the interactions of PFOA, a toxic industrial waste in water, and its interactions with COFs synthesized by Trabolsi Lab.
The selective adsorption and removal of dye molecules from industrial pollutant water remain one of the main challenges in some of the major manufacturing processes such as textile, pharmaceuticals. However, the methods currently applied in the industry to the removal of dye molecules are not efficient. Therefore, the application of covalent organic frameworks (COF) such as calixarene is a novel approach which is being explored. We use computational simulations to understand the selective adsorption of different types of dyes on COFs. The advancement of this project so far empowers us to use these tools to predict the experimental outcomes, saving a lot of time, money and efforts. Also, these tools can be easily transferable to use in the industry to resolve issues in the selective adsorption or filtration processes using polymers or membranes. Fig 3 Shows ligand sampling approach we developed employing metadynamics for that purpose.
Fig 1. I2 distributions in the CX4-NS polymer matrix from Grand Canonical Monte Carlo simulations. (a) A snapshot from simulations. Yellow ball-and-stick figures represent instantaneous I2 vapor molecules. (b) top and (c) side views of the construct. The 3D density map of I2 is shown in surface representation. The gray isosurface corresponds to iodine density of ρ > 20ρb, where ρ b is the bulk density of ρ > 20ρ2 vapor, transparent yellow represents ρ > 100ρb,and solid yellow represents ρ > 200ρb.
Fig2. Molecular modelling shows how PFAO molecules in water preferentially bind to calixerene based COF polymers.
Fig 3. A representative structure of dye-COF model for Metadynamics simulations setup and the choice of two collective variables used to bias the sampling. Z is the distance along z-axis of the center of mass (COM) of dye molecule and that of the phenolic oxygens (orange), r is the Euclidian distance in x, y plane between COM of dye and COM of phenolic oxygens (d) Free energy landscape of COF-dye binding.
References:
Shetty, D, Skorjanc, T., Raya, J., Sharma, SK, Jahovic, I., Polychronopoulou, K., Asfari, Z., Han, DS., Dewage, S., Olsen, JO., Jagannathan, R., Kirmizialtin, S., and Trabolsi A. “Calix[4]arene-Based Porous Organic Nanosheets”, ACS Appl. Mater. Interfaces, 10, 17359, (2018)
Skorjanc, T., Shetty, D., Sharma, S., Raya, S., Traboulsi, H., Han, DS., Lalla, J., Newlon, R., Jagannathan, R., Kirmizialtin, S., Olsen, JC., Trabolsi, A., “Redox‐responsive Covalent Organic Nanosheets from Viologens and Calix [4] arene for Iodine and Toxic Dye Capture”, Chem. Eur. J., 24, 1,(2018)