Introduction_Amit
Cation mediated nucleic acid interactions
Highly negatively charged bio-polyelectrolytes such as nucleic acids (both DNA and RNA) cannot exert their biological function in any viable organism without the presence of metal ions such as sodium, potassium, magnesium and other multivalent ions. The intricate interplay between the negatively charged backbone and the cations in solution is key to understand how these molecules fold and function in different environments.
DNA CONDENSATION
Motivated by the biological significance of genome packaging -particularly in simple systems such as virus, bacteriophage and sperms, cations-mediated DNA-DNA interaction has attracted substantial interest both from theorists and experimentalist. Condensation of DNA induced by multivalent ions has been studied for many years. Divalent alkali cations (Mg2+) are already known to play important roles in nucleic acid interaction but divalent ions induced condensation are not reported yet. Condensation has been reported for other polyelectrolyte as well, including actin and microtubules. Although a lot of effort has been done to elucidate the mechanism involved in stabilizing the condensed state but the detailed atomic level mechanism of condensation is still unclear.
Fig 1. Top panel shows the Mg ion distribution along 1D and bottom panel shows the Mg ion distribution along x-y axis.
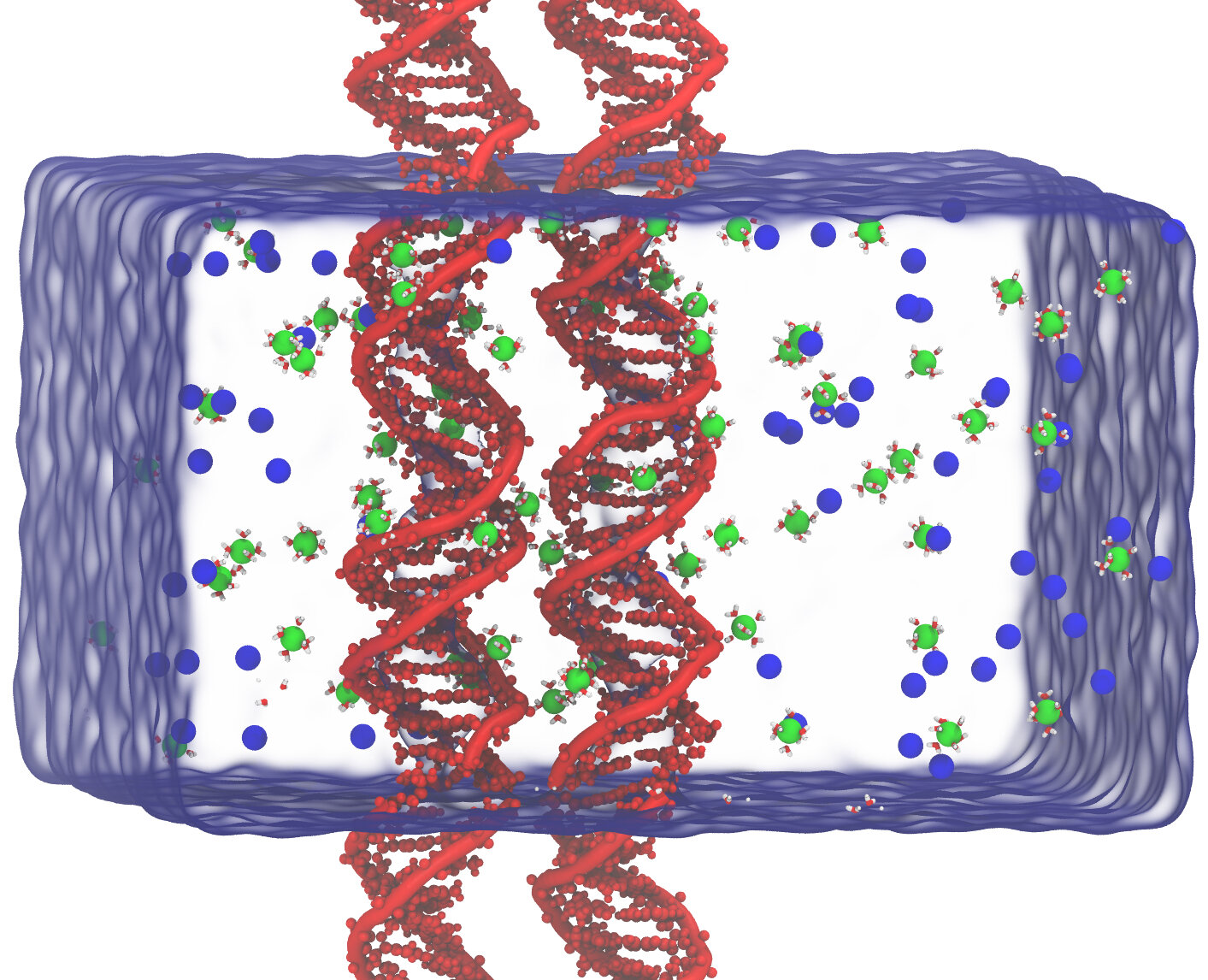
Fig 2. Simulation box and the two DNA duplexes positioned parallel to each other with an interhelical distance of d. Green and blue spheres represent hexa-hydrated Mg(H2O)6and Cl respectively.
Cation-induced condensation cannot be explained with mean-field-theory that always predict a repulsive interaction between like-charged polyelectrolytes. Recent advances in the physics of strongly interacting systems go beyond the classical framework and it is now well established that dynamic correlation of cations gives rise to an attractive force. In theoretical modeling, DNA is usually treated as a uniformly charged cylinder, the counterions as point charges, and water as a continuous dielectric medium. In dense system such as DNA condensation, a molecular description is necessary for an understanding of the condensation phenomenon. This can be achieved with the molecular dynamics (MD) computer simulation method.
In our recent work, we report the first observation of sequence-dependent dsDNA condensation by divalent alkali cations using MD simulations. We also propose a new mechanism of sequence-directed DNA condensation, where consecutive adenine bases enhance the cation groove binding and the exposed cations are spatially correlated with the phosphate groups from neighboring DNA to elicit attraction.